
Chemistry, 13.12.2021 15:50 daevinepetersopa86on
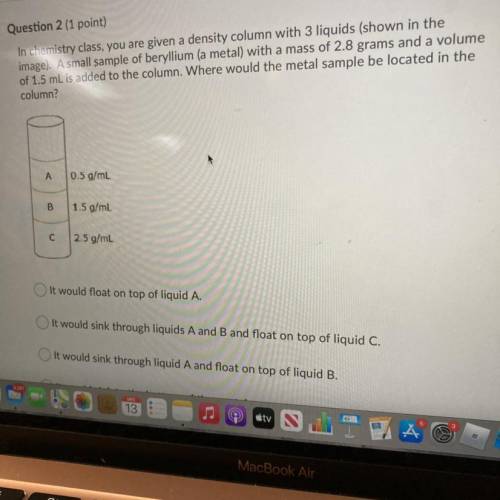
In chemistry class, you are given a density column with 3 liquids (shown in the
image). A small sample of beryllium (a metal) with a mass of 2.8 grams and a volume
of 1.5 mL is added to the column. Where would the metal sample be located in the
column?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
In chemistry class, you are given a density column with 3 liquids (shown in the
image). A small sa...
Questions


History, 24.08.2019 19:10

Health, 24.08.2019 19:10


Chemistry, 24.08.2019 19:10



Health, 24.08.2019 19:10


Mathematics, 24.08.2019 19:10




Computers and Technology, 24.08.2019 19:10



Mathematics, 24.08.2019 19:10


History, 24.08.2019 19:10




