
The picture below shows a soil texture triangle.
David plans to grow corn on his farm. Corn grows best in clay loam soil. Which of these is the most likely soil sample that David would select to grow corn?
Question 16 options:
sample containing 30 percent sand, 35 percent clay, and 35 percent silt
sample containing 25 percent sand, 15 percent clay, and 60 percent silt
sample containing 55 percent sand, 30 percent clay, and 15 percent silt
sample containing 40 percent sand, 15 percent clay, and 45 percent silt
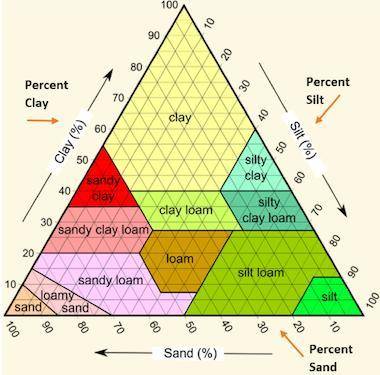

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1
You know the right answer?
The picture below shows a soil texture triangle.
David plans to grow corn on his farm. Corn grows...
Questions

Mathematics, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01

Biology, 29.09.2020 20:01


Mathematics, 29.09.2020 20:01

Physics, 29.09.2020 20:01

Computers and Technology, 29.09.2020 20:01


History, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01


History, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01





Mathematics, 29.09.2020 20:01



