Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
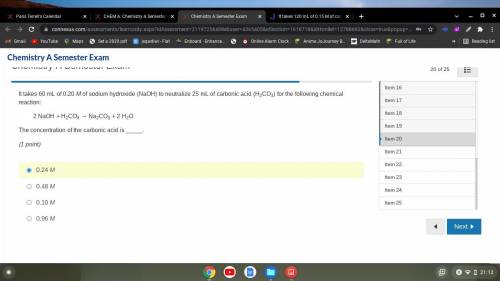
It takes 60 mL of 0.20 M of sodium hydroxide (NaOH) to neutralize 25 mL of carbonic acid (H2CO3) for...
Questions


English, 04.05.2021 04:00


Chemistry, 04.05.2021 04:00

Spanish, 04.05.2021 04:00

Mathematics, 04.05.2021 04:00


Mathematics, 04.05.2021 04:00

Mathematics, 04.05.2021 04:10





Mathematics, 04.05.2021 04:10

Mathematics, 04.05.2021 04:10

Mathematics, 04.05.2021 04:10

Geography, 04.05.2021 04:10



English, 04.05.2021 04:10