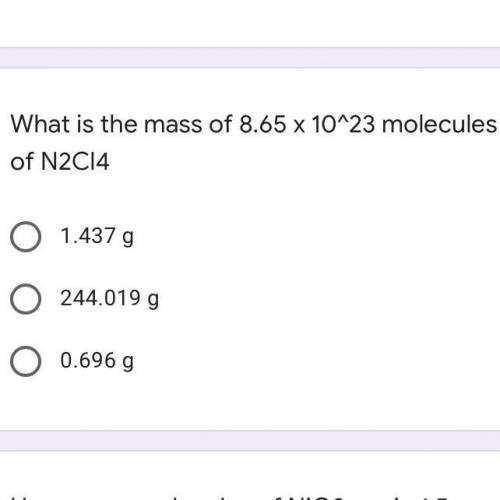
What is the mass of 8.65 x 10^23 molecules of N2Cl4
A. 1.437 g
B. 244.019 g
C. 0.696 g...

Chemistry, 14.12.2021 21:50 baeethtsadia
What is the mass of 8.65 x 10^23 molecules of N2Cl4
A. 1.437 g
B. 244.019 g
C. 0.696 g

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
Questions


Mathematics, 12.10.2019 20:50


Mathematics, 12.10.2019 20:50



Biology, 12.10.2019 20:50










Mathematics, 12.10.2019 21:00

Chemistry, 12.10.2019 21:00


Health, 12.10.2019 21:00



