
Chemistry, 14.12.2021 22:00 CaptainKiller528
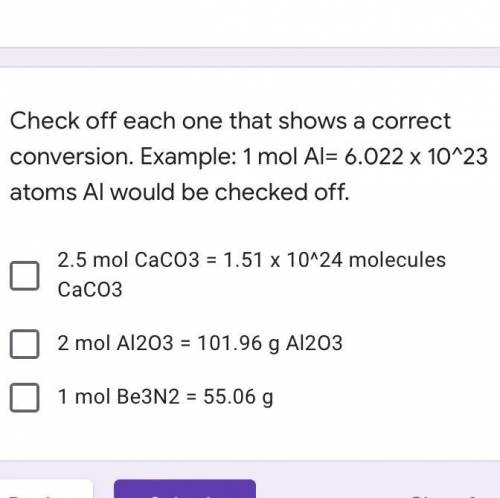
Check off each one that shows a correct conversion. Example: 1 mol Al= 6.022 x 10^23 atoms Al would be checked off.
A. 2.5 mol CaCO3 = 1.51 x 10^24 molecules CaCO3
B. 2 mol Al2O3 = 101.96 g Al2O3
C. 1 mol Be3N2 = 55.06 g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Check off each one that shows a correct conversion. Example: 1 mol Al= 6.022 x 10^23 atoms Al would...
Questions

Mathematics, 01.12.2020 19:20





Mathematics, 01.12.2020 19:20

History, 01.12.2020 19:20



Health, 01.12.2020 19:20

Mathematics, 01.12.2020 19:20


History, 01.12.2020 19:20

Business, 01.12.2020 19:20

English, 01.12.2020 19:20


Advanced Placement (AP), 01.12.2020 19:20





