
Chemistry, 15.12.2021 02:30 Lenaaa2019
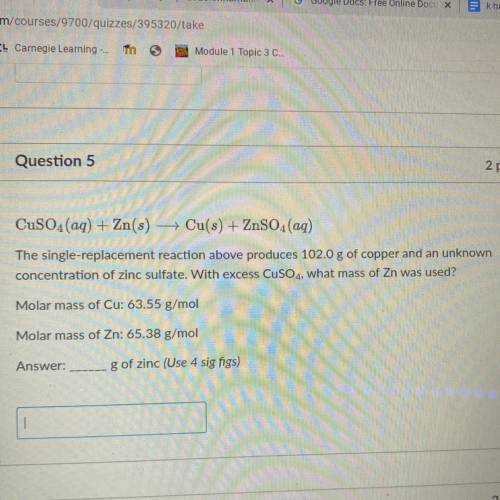
CuSO4(aq) + Zn(s) — Cu(s) + ZnSO4(aq)
The single-replacement reaction above produces 102.0 g of copper and an unknown
concentration of zinc sulfate. With excess CuSO4, what mass of Zn was used?
Molar mass of Cu: 63.55 g/mol
Molar mass of Zn: 65.38 g/mol
g of zinc (Use 4 sig figs)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
CuSO4(aq) + Zn(s) — Cu(s) + ZnSO4(aq)
The single-replacement reaction above produces 102.0 g of co...
Questions


Geography, 30.12.2019 02:31

Mathematics, 30.12.2019 02:31






Mathematics, 30.12.2019 02:31


History, 30.12.2019 02:31



Mathematics, 30.12.2019 02:31



English, 30.12.2019 02:31

Mathematics, 30.12.2019 02:31




