
Chemistry, 15.12.2021 03:50 juliawatakip5fmg7
The formula for the ionic compound formed between calciumions and chloride ions is CaCl2. What does this formula tell you about the compound? Which one is it :)
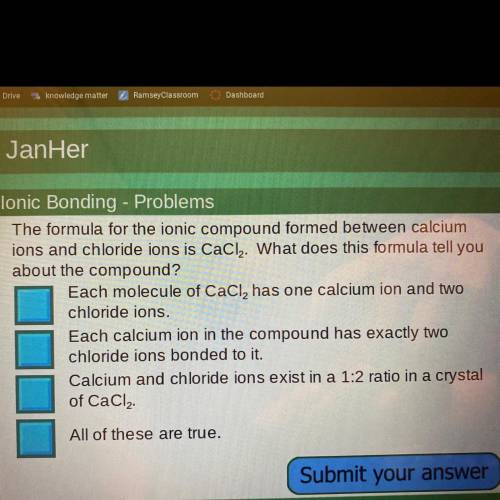

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
The formula for the ionic compound formed between calciumions and chloride ions is CaCl2. What does...
Questions



Mathematics, 20.07.2019 22:40

Mathematics, 20.07.2019 22:40



Social Studies, 20.07.2019 22:40







Geography, 20.07.2019 22:40



Mathematics, 20.07.2019 22:40



Geography, 20.07.2019 22:40




