
Chemistry, 15.12.2021 23:20 juelchasse
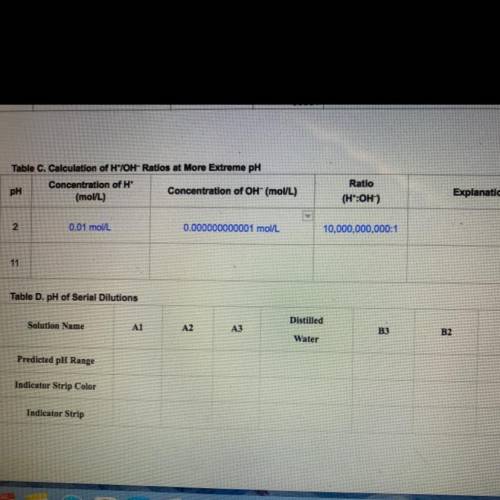
In Step 5, you will calculate H*/OH- ratios for more
extreme pH solutions. Find the concentration of H* ions
to OH- ions listed in Table B of your Student Guide for a
solution at a pH = 2. Then divide the H* concentration by
the OH concentration. Record these concentrations and
ratio in Table C.
I know the answer I just don’t know what to put for the explanation on the table.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
In Step 5, you will calculate H*/OH- ratios for more
extreme pH solutions. Find the concentration...
Questions


Chemistry, 01.09.2019 08:50

History, 01.09.2019 08:50


English, 01.09.2019 08:50

Physics, 01.09.2019 08:50

History, 01.09.2019 08:50

History, 01.09.2019 08:50

English, 01.09.2019 08:50





History, 01.09.2019 08:50


Mathematics, 01.09.2019 08:50

Business, 01.09.2019 08:50

Mathematics, 01.09.2019 08:50


Mathematics, 01.09.2019 08:50



