
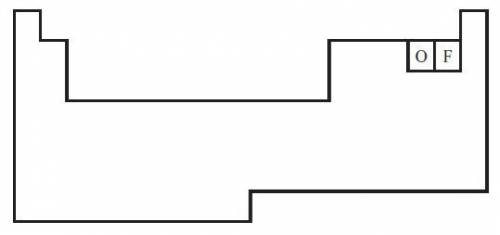
The diagram below shows a partial periodic table.
The electron configuration of oxygen is 1s2 2s2 2p4. On the periodic table, fluorine is one space to the right of oxygen. Which of the following electron configurations represents fluorine?
1s2 2s2 2p3
1s2 2s2 2p6 1s2 3s2 3p3
1s2 2s2 2p5

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2


Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
The diagram below shows a partial periodic table.
The electron configuration of oxygen is 1s2 2s2...
Questions


English, 16.12.2021 07:30

Mathematics, 16.12.2021 07:30


English, 16.12.2021 07:30

Mathematics, 16.12.2021 07:30

Mathematics, 16.12.2021 07:30

Mathematics, 16.12.2021 07:30

Mathematics, 16.12.2021 07:30






English, 16.12.2021 07:30


Engineering, 16.12.2021 07:30

Computers and Technology, 16.12.2021 07:30

Mathematics, 16.12.2021 07:30



