
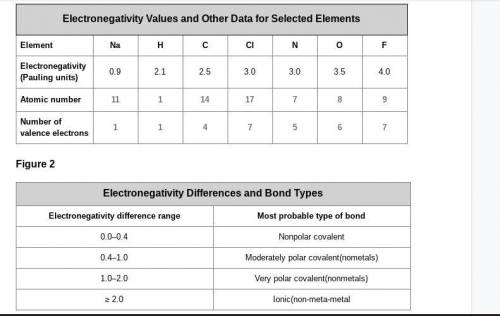
A cesium (Cs) atom has an atomic number of 55 and one electron in its outer shell. Considering the data in Figure 1 and Figure 2, what electronegativity value would you expect it to have, and what kind of bond is it likely to form with a chlorine atom? Explain your reasoning.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
A cesium (Cs) atom has an atomic number of 55 and one electron in its outer shell. Considering the d...
Questions


English, 25.01.2020 12:31

History, 25.01.2020 12:31

History, 25.01.2020 12:31

English, 25.01.2020 12:31

History, 25.01.2020 12:31



Mathematics, 25.01.2020 12:31



History, 25.01.2020 12:31




Biology, 25.01.2020 12:31

Health, 25.01.2020 12:31

Mathematics, 25.01.2020 12:31


Mathematics, 25.01.2020 12:31



