
PLZ HELP Stoichiometry Calculation
Imagine that you are solving the following stoichiometry problem:
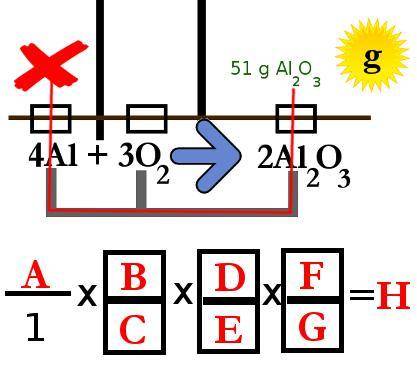
When aluminum oxidizes in air, it forms aluminum oxide (Al2O3):
4Al + 3O2 --> 2Al2O3 if a 51g sheet of aluminum oxide formed completely in excess oxygen, how many grams of aluminum were oxidized? Using the magic mole method, you diagram the problem, map out your solution route, and convert your diagram to an equation.
Use this diagram to answer the problems below.
(diagram included
2.What units go in spot C in the equation above?
mol Al2O3. mol. g. g Al2O3
3.What units go in spot B in the equation above?
g Al. mol Al2O3. mol Al. g Al2O3
4.What number goes in spot D in the equation? Enter the number only without any units
5.What number goes in spot E in the equation above? (Enter the number only

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
PLZ HELP Stoichiometry Calculation
Imagine that you are solving the following stoichiometry proble...
Questions

Chemistry, 12.10.2020 02:01


Mathematics, 12.10.2020 02:01

Chemistry, 12.10.2020 02:01


English, 12.10.2020 02:01


Mathematics, 12.10.2020 02:01





Arts, 12.10.2020 02:01


Mathematics, 12.10.2020 02:01







