
Chemistry, 16.12.2021 23:10 coollid876
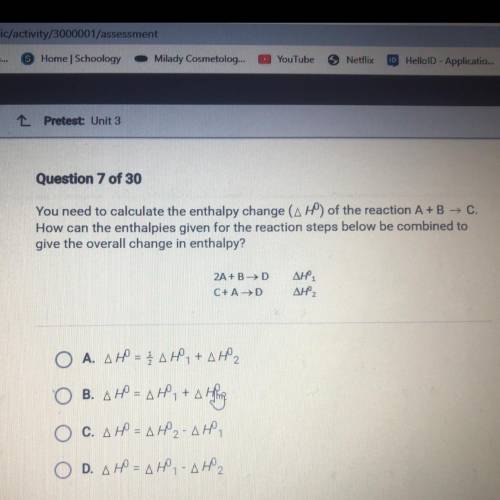
You need to calculate the enthalpy change (AHO) of the reaction A+B — C.
How can the enthalpies given for the reaction steps below be combined to
give the overall change in enthalpy?
2A + B HD
С+ AHD
Дне.
ДНР,
ОА. ДР – ДЕ, +д,
ОВ. ДР - ДР. +д Н.
=
Ос. дф = ду-дю,
D. др = два дно,

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
You need to calculate the enthalpy change (AHO) of the reaction A+B — C.
How can the enthalpies gi...
Questions

Mathematics, 18.11.2021 14:00

Mathematics, 18.11.2021 14:00

English, 18.11.2021 14:00

Computers and Technology, 18.11.2021 14:00




Spanish, 18.11.2021 14:00

English, 18.11.2021 14:00


Arts, 18.11.2021 14:00



Advanced Placement (AP), 18.11.2021 14:00






English, 18.11.2021 15:00



