
Chemistry, 17.12.2021 05:30 chasechevy13
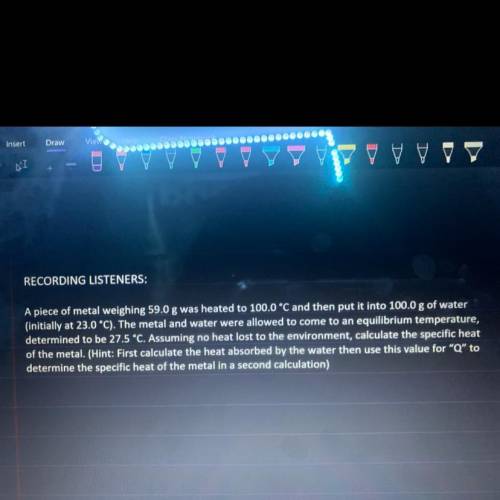
A piece of metal weighing 59.0 g was heated to 100 C and then put into 100.0 g of water (initially at 23.0 C) the metal and water were allowed to come to an equilibrium temperature, determined to be 27.5 C. Assuming no heat lost to the environment, calculate the specific heat of the metal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1


Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
A piece of metal weighing 59.0 g was heated to 100 C and then put into 100.0 g of water (initially a...
Questions


Mathematics, 12.01.2021 20:30


Arts, 12.01.2021 20:30


Mathematics, 12.01.2021 20:30

English, 12.01.2021 20:30

Mathematics, 12.01.2021 20:30

Mathematics, 12.01.2021 20:30


History, 12.01.2021 20:30


Mathematics, 12.01.2021 20:30

Health, 12.01.2021 20:30

Physics, 12.01.2021 20:30


Social Studies, 12.01.2021 20:30

Mathematics, 12.01.2021 20:30


History, 12.01.2021 20:30



