
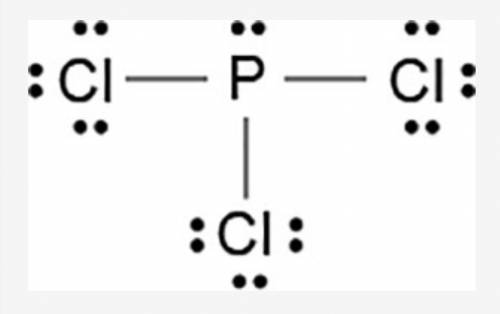
The Lewis dot model of a molecule is shown.
A visual diagram of a PCl3 molecule is shown. Phosphorous is the central atom with a horizontal line connecting to each of the three Chlorine atoms around it. Phosphorous has a pair of dots on it. Each of the three chlorine atoms have a pair of three dots on it.
Based on the model, which of the following is true?
The electronegativity difference between phosphorous and chlorine is greater than 1.7.
Each chlorine has three non-bonded pairs and one bonded pair of electrons.
Phosphorous has three non-bonded pairs and one bonded pair of electrons.
Phosphorous has three valence electrons in the outermost energy level.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Monkeys and bats have similar bone structure in their forelimbs. however, monkeys have longer forelimbs to use for climbing and swinging in trees. bats have shorter forelimbs to use for flight. which term best describes how monkey and bat forelimbs are related to each other? a. homologous b. embryonic c. analogous d. vestigial
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3
You know the right answer?
The Lewis dot model of a molecule is shown.
A visual diagram of a PCl3 molecule is shown. Phosphor...
Questions


Mathematics, 21.01.2021 22:10




Computers and Technology, 21.01.2021 22:10
















