
Chemistry, 20.12.2021 22:10 jesuscruzm2020
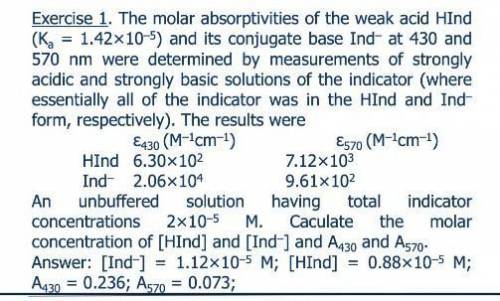
The molar absorptivities at 430 and 570 nm of the weak acid HIn (Ka = 1.42 ? 10-5) and its conjugate base In- were determined by measurements of strongly acidic and strongly basic solutions of the indicator. Under this conditions, essentially all of the indicator was in the HIn and In- form, respectively. The molar ab-sorptivities of HIn and In- at 430 nm and 570 nm were 6.30 ? 102 and 7.12 ? 103, and 2.06 ? 104 and 9.61 ? 102, respectively. Calculate absorbance data for unbuffered solutions that have total indicator concentrations ranging from 2 ? 10-5 to 16 ? 10-5 M.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1


Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
The molar absorptivities at 430 and 570 nm of the weak acid HIn (Ka = 1.42 ? 10-5) and its conjugate...
Questions

English, 06.05.2021 21:00

English, 06.05.2021 21:00



Mathematics, 06.05.2021 21:00

Geography, 06.05.2021 21:00

Mathematics, 06.05.2021 21:00

English, 06.05.2021 21:00

Chemistry, 06.05.2021 21:00

Physics, 06.05.2021 21:00

Computers and Technology, 06.05.2021 21:00

History, 06.05.2021 21:00

Mathematics, 06.05.2021 21:00



Health, 06.05.2021 21:00


Chemistry, 06.05.2021 21:00

Mathematics, 06.05.2021 21:00

History, 06.05.2021 21:00



