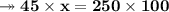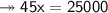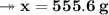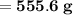Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
Knowing that the solubility of a salt at 80°C is 45g/100 g of H, O, calculate the mass of water requ...
Questions


Mathematics, 02.07.2019 04:00

Social Studies, 02.07.2019 04:00

SAT, 02.07.2019 04:00


Mathematics, 02.07.2019 04:00

Mathematics, 02.07.2019 04:00


Mathematics, 02.07.2019 04:00



English, 02.07.2019 04:00

Mathematics, 02.07.2019 04:00

Mathematics, 02.07.2019 04:00

Biology, 02.07.2019 04:00



Mathematics, 02.07.2019 04:00

Geography, 02.07.2019 04:00

Chemistry, 02.07.2019 04:00

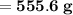 .
. 45g of salt
45g of salt  100g of H₂O
100g of H₂O