
Chemistry, 27.12.2021 03:20 meababy2009ow9ewa
Burning 1.6 grams of substance M requires 6.4 grams of oxygen to produce carbon dioxide (CO2) and water (H2O) according to the mass ratio:
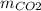 :
: 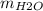 = 11 : 9
= 11 : 9
Calculate the mass of CO2 and H2O produced.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
Burning 1.6 grams of substance M requires 6.4 grams of oxygen to produce carbon dioxide (CO2) and wa...
Questions

Mathematics, 10.07.2019 23:50




Mathematics, 11.07.2019 00:00

English, 11.07.2019 00:00

Mathematics, 11.07.2019 00:00


Mathematics, 11.07.2019 00:00









English, 11.07.2019 00:00


Biology, 11.07.2019 00:00



