Answer the following questions.
P1) Matching.
Complete the table with the data from the lab....

Chemistry, 28.12.2021 05:50 dajeourcrazy15
Answer the following questions.
P1) Matching.
Complete the table with the data from the lab. First, calculate and enter the mole ratio in the chart, then enter the volumes of the precipitates.
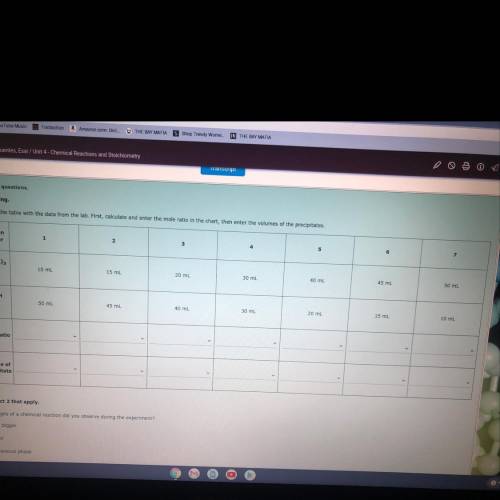

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
You know the right answer?
Questions


Computers and Technology, 30.06.2021 15:30

Mathematics, 30.06.2021 15:30

Mathematics, 30.06.2021 15:30


Computers and Technology, 30.06.2021 15:30

Mathematics, 30.06.2021 15:30

Social Studies, 30.06.2021 15:30

Biology, 30.06.2021 15:30

Chemistry, 30.06.2021 15:30

Social Studies, 30.06.2021 15:30

Computers and Technology, 30.06.2021 15:30

Mathematics, 30.06.2021 15:30



Mathematics, 30.06.2021 15:30

Mathematics, 30.06.2021 15:30

Biology, 30.06.2021 15:30

Mathematics, 30.06.2021 15:30

Biology, 30.06.2021 15:30



