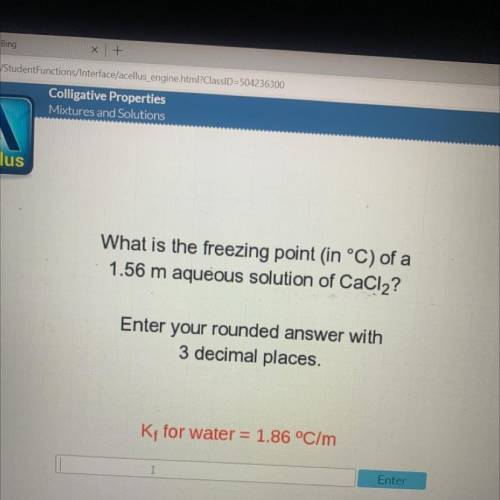
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded...

Chemistry, 02.01.2022 14:00 edimilperdomo
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded answer with
3 decimal places.
Kt for water = 1.86 °C/m

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
You know the right answer?
Questions

Mathematics, 31.01.2020 08:04


English, 31.01.2020 08:04

Geography, 31.01.2020 08:04


Mathematics, 31.01.2020 08:04




History, 31.01.2020 08:04




World Languages, 31.01.2020 08:04


Mathematics, 31.01.2020 08:04




Mathematics, 31.01.2020 08:04



