Which example has 1.2×10^24 hydrogen atoms?
2 moles of ammonia (NH3)
2 moles of hydroge...

Chemistry, 05.01.2022 20:50 lineaeriksen
Which example has 1.2×10^24 hydrogen atoms?
2 moles of ammonia (NH3)
2 moles of hydrogen gas (H2)
1 mole of water (H2O)
1 mole of methane (CH4)
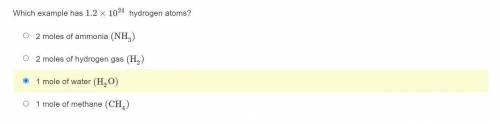

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3


Chemistry, 23.06.2019 12:30
Atriple covalent bond involves two atoms sharing three pairs of electrons. true false
Answers: 2
You know the right answer?
Questions


Physics, 26.05.2021 01:20


Mathematics, 26.05.2021 01:20

History, 26.05.2021 01:20

History, 26.05.2021 01:20

Biology, 26.05.2021 01:20



Mathematics, 26.05.2021 01:20

Mathematics, 26.05.2021 01:20


Biology, 26.05.2021 01:20

Mathematics, 26.05.2021 01:20


Mathematics, 26.05.2021 01:20






