
Chemistry, 06.01.2022 02:30 priceisright11401
PLEASE HELP ASAP WILL GIVE BRAINLIEST
(dont give chegg link pls)
How much heat is required to convert 150 g of liquid H2O at 43.5 °C into steam at 130 °C?
Specific heat of liquid water is 4.18 J/g oC.
Specific heat of water vapor is 1.84 J/g oC.
ΔHvap = 40.67 kJ/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
PLEASE HELP ASAP WILL GIVE BRAINLIEST
(dont give chegg link pls)
How much heat is req...
How much heat is req...
Questions




History, 04.06.2020 10:57

Mathematics, 04.06.2020 10:57


Mathematics, 04.06.2020 10:57




Chemistry, 04.06.2020 10:57


English, 04.06.2020 10:57

Mathematics, 04.06.2020 10:57

Mathematics, 04.06.2020 10:57

Mathematics, 04.06.2020 10:57

Biology, 04.06.2020 10:57

Mathematics, 04.06.2020 10:57


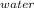 *ΔT
*ΔT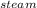 * ΔT
* ΔT

