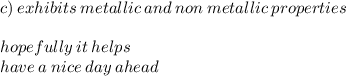Chemistry, 09.01.2022 05:40 anonymous176
4. Pure silicon is chemically classified as a metalloid because silicon
A) is malleable and ductile
B) is an excellent conductor of heat and electricity
C) exhibits metallic and nonmetallic properties D) none of the above

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1
You know the right answer?
4. Pure silicon is chemically classified as a metalloid because silicon
A) is malleable and ductil...
Questions


History, 18.11.2020 17:50

Mathematics, 18.11.2020 17:50


Mathematics, 18.11.2020 17:50

Health, 18.11.2020 17:50

History, 18.11.2020 17:50

Mathematics, 18.11.2020 17:50


Business, 18.11.2020 17:50

English, 18.11.2020 17:50


Mathematics, 18.11.2020 17:50

Mathematics, 18.11.2020 17:50

Mathematics, 18.11.2020 17:50

Mathematics, 18.11.2020 17:50

History, 18.11.2020 17:50

Mathematics, 18.11.2020 17:50

Mathematics, 18.11.2020 17:50

Biology, 18.11.2020 17:50