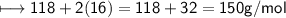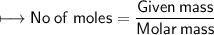ASAP
SnO2 (s) + 2 H2(g) → Sn (s) + 2 H2O (1)
What mass of water is produce when 80.8g of SnO...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Questions



Chemistry, 05.05.2020 04:33


History, 05.05.2020 04:33

Social Studies, 05.05.2020 04:33





History, 05.05.2020 04:33

Mathematics, 05.05.2020 04:33


Mathematics, 05.05.2020 04:33

Computers and Technology, 05.05.2020 04:33



Mathematics, 05.05.2020 04:33