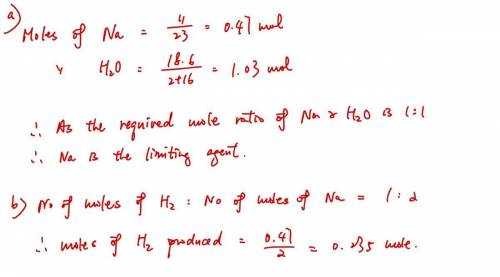A sample of 11.0 g of sodium is reacted with 18.6 g of water to produce sodium hydroxide and hydrogen gas. Using the balanced equation below, predict which is the limiting reactant and the maximum amount in moles of hydrogen gas that can be produced. 2Na+ 2H2O + 2NaOH + H2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3
You know the right answer?
A sample of 11.0 g of sodium is reacted with 18.6 g of water to produce sodium hydroxide and hydroge...
Questions


Biology, 20.05.2021 08:10



Spanish, 20.05.2021 08:10

Mathematics, 20.05.2021 08:10

English, 20.05.2021 08:10


Computers and Technology, 20.05.2021 08:10

English, 20.05.2021 08:10




Mathematics, 20.05.2021 08:10


English, 20.05.2021 08:10

History, 20.05.2021 08:10


Mathematics, 20.05.2021 08:10