

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1
You know the right answer?
When calcium carbonate is heated, it decomposes to produce calcium oxide and carbon dioxide, as show...
Questions



Computers and Technology, 31.07.2019 19:00

Mathematics, 31.07.2019 19:00

Computers and Technology, 31.07.2019 19:00

Chemistry, 31.07.2019 19:00

English, 31.07.2019 19:00

Chemistry, 31.07.2019 19:00

History, 31.07.2019 19:00

Biology, 31.07.2019 19:00



Mathematics, 31.07.2019 19:00

Mathematics, 31.07.2019 19:00

Advanced Placement (AP), 31.07.2019 19:00

Mathematics, 31.07.2019 19:00


Biology, 31.07.2019 19:00

Mathematics, 31.07.2019 19:00

Mathematics, 31.07.2019 19:00

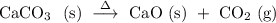 ,
,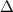 , above the chemical reaction arrow denotes that heat is applied to make the reaction proceed in the direction of the arrow.
, above the chemical reaction arrow denotes that heat is applied to make the reaction proceed in the direction of the arrow.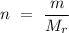 ,
,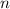 is the number of moles,
is the number of moles, 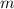 is the mass of the chemical substance and
is the mass of the chemical substance and 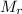 is the molar mass of the chemical substance. We first need to identify the molar mass of calcium carbonate,
is the molar mass of the chemical substance. We first need to identify the molar mass of calcium carbonate,![M_{r} \, [\text{CaCO}_{3}] \ = \ A_{r} \, [\text{Ca}] \ + \ A_{r} \, [\text{C}] \ + \ 3 \, \times \, A_{r} \, [\text{O}] \\ \\ M_{r} \, [\text{CaCO}_{3}] \ = \ 40.078 \ + \ 12.011 \ + \ 3 \, \times \, 15.999 \\ \\ M_{r} \, [\text{CaCO}_{3}] \ = \ 100.086 \ \text{g mol}^{-1}](/tpl/images/2615/0156/37e0e.png)
 .
.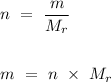 .
.![M_{r} \, [\text{CO}_{2}] \ = \ A_{r} \, [\text{C}] \ + \ 2 \, \times \, A_{r} \, [\text{O}] \\ \\ M_{r} \, [\text{CO}_{2}] \ = \ (12.011 \ + \ 2 \, \times \, 15.999) \ \text{g mol}^{-1} \\ \\ M_{r} \, [\text{CO}_{2}] \ = \ 44.009 \ \text{g mol}^{-1}](/tpl/images/2615/0156/8567d.png)




