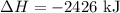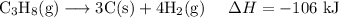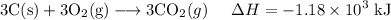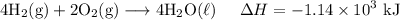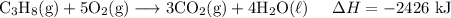H2 (g)+12O2 (g)→H2O (l) ΔH=−286 kJ

Chemistry, 18.01.2022 08:50 monkeyrose1999
Study the reactions.
C (s)+O2 (g)→CO2 (g) ΔH=−394 kJ
H2 (g)+12O2 (g)→H2O (l) ΔH=−286 kJ
3C (s)+4H2 (g)→C3H8 (g) ΔH=106 kJ
Target Reaction:
C3H8 (g)+5O2 (g)→3CO2 (g)+4H2O (l)ΔH= ?
What is the enthalpy change of the target reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Study the reactions.
C (s)+O2 (g)→CO2 (g) ΔH=−394 kJ
H2 (g)+12O2 (g)→H2O (l) ΔH=−286 kJ
H2 (g)+12O2 (g)→H2O (l) ΔH=−286 kJ
Questions


History, 19.12.2019 18:31

English, 19.12.2019 18:31

History, 19.12.2019 18:31


Mathematics, 19.12.2019 18:31


English, 19.12.2019 18:31

Mathematics, 19.12.2019 18:31


Chemistry, 19.12.2019 18:31

Mathematics, 19.12.2019 18:31

Mathematics, 19.12.2019 18:31

English, 19.12.2019 18:31



English, 19.12.2019 18:31


Mathematics, 19.12.2019 18:31