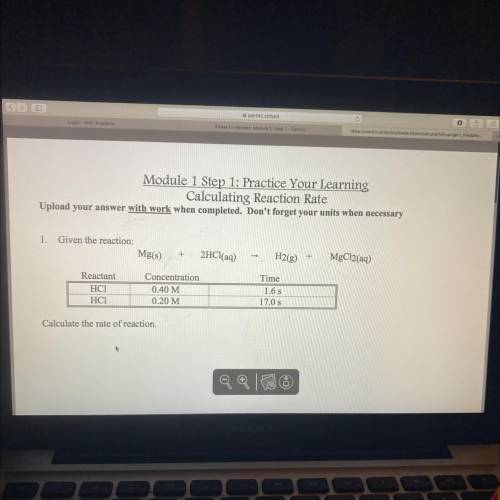
Module 1 Step 1: Practice Your Learning
Calculating Reaction Rate
Upload your answer with wo...

Chemistry, 20.01.2022 03:50 jaydenbomkamp6084
Module 1 Step 1: Practice Your Learning
Calculating Reaction Rate
Upload your answer with work when completed. Don't forget your units when necessary
1.
Given the reaction:
Mg(s)
+
2HCl(aq)
-
H2(g)
+
MgCl2(aq)
Reactant
HCI
НСІ
Concentration
0.40 M
0.20 M
Time
1.6 s
17.0 s
Calculate the rate of reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
Questions




Social Studies, 12.01.2021 17:50

Arts, 12.01.2021 17:50

History, 12.01.2021 17:50

Mathematics, 12.01.2021 17:50

Social Studies, 12.01.2021 17:50


History, 12.01.2021 17:50


English, 12.01.2021 17:50

Mathematics, 12.01.2021 17:50



Mathematics, 12.01.2021 17:50

Physics, 12.01.2021 17:50

Advanced Placement (AP), 12.01.2021 17:50

History, 12.01.2021 17:50

Biology, 12.01.2021 17:50



