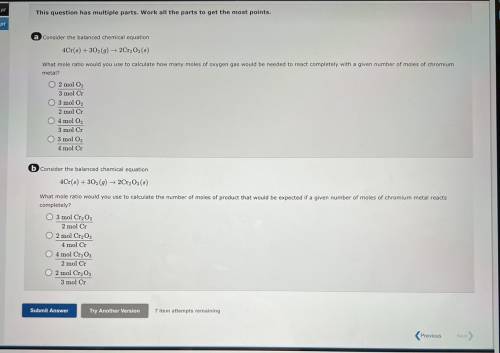
A consider the balanced chemical equation
4Cr(6) + 30(9) 2Cr, Os(8)
What mole ratio would yo...

Chemistry, 21.01.2022 01:00 aliciaa101
A consider the balanced chemical equation
4Cr(6) + 30(9) 2Cr, Os(8)
What mole ratio would you use to calculate how many moles of oxygen gas would be needed to react completely with a given number of moles of chromium
metal?
O 2 mol O,
3 mol Cr
O 3 mol O2
2 mol Cr
04 mol O
3 mol C
O 3 mol O2
4 mol Cr
6 Consider the balanced chemical equation
4Cr(6) +302 (9) ► 2Cr, Os()
What mole ratio would you use to calculate the number of moles of product that would be expected if a given number of moles of chromium metal reacts
completely?
O 3 mol Cr2O3
2 mol C
O 2 mol CrgO;
4 mol Cr
O 4 mol Cr2O3
2 mol Cr
O 2 mol Cryo,
3 mol Cr

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1
You know the right answer?
Questions



History, 29.01.2021 02:00


Health, 29.01.2021 02:00



Medicine, 29.01.2021 02:00



Mathematics, 29.01.2021 02:00


Mathematics, 29.01.2021 02:00



Business, 29.01.2021 02:00

Mathematics, 29.01.2021 02:00

Social Studies, 29.01.2021 02:00

Chemistry, 29.01.2021 02:00

Mathematics, 29.01.2021 02:00



