HELP PLEASE
Use the following steps to balance the redox reaction below:
Mg + Au+ Mg2+ + Au<...

Chemistry, 23.01.2022 14:00 sarahgrindstaff123
HELP PLEASE
Use the following steps to balance the redox reaction below:
Mg + Au+ Mg2+ + Au
a. Write the oxidation and reduction half-reactions. Make sure each half-reaction is balanced for number of atoms
and charge. (3 points)
b. Multiply each half reaction by the correct number in order to balance charges for the two half reactions
c. Add the equations and simplify to get a balanced equation
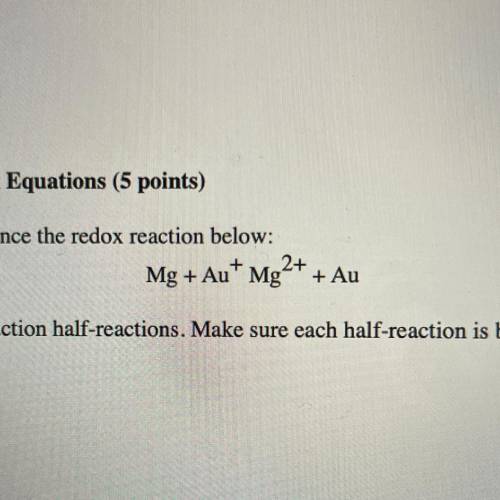

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

You know the right answer?
Questions

English, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31

Health, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31

Biology, 14.12.2019 14:31



Biology, 14.12.2019 14:31

English, 14.12.2019 14:31

History, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31





History, 14.12.2019 14:31



