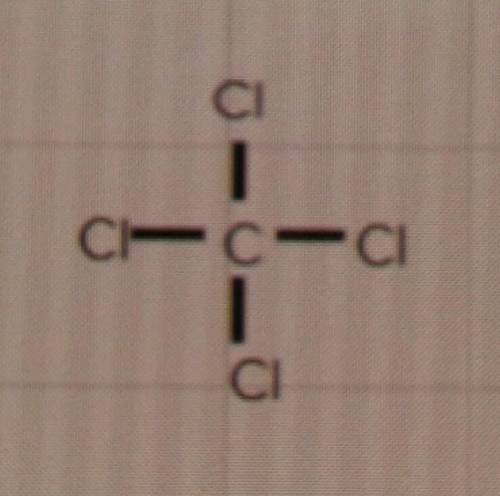Chemistry, 23.01.2022 20:50 Ilcienne6590
Hi! Can someone help me with this???
The question: Will these compounds form single, double or triple bonds?
d) CCℓ4
I'm just a bit confused because the Lewis structures look like it would be a 4 bond, but I don't know if thats even a thing. My only options are single, double or triple.
Thank you!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Hi! Can someone help me with this???
The question: Will these compounds form single, double or tri...
Questions





Social Studies, 29.12.2020 01:20


History, 29.12.2020 01:20

English, 29.12.2020 01:20


Mathematics, 29.12.2020 01:20

Law, 29.12.2020 01:20


Health, 29.12.2020 01:20




Mathematics, 29.12.2020 01:20

Mathematics, 29.12.2020 01:20