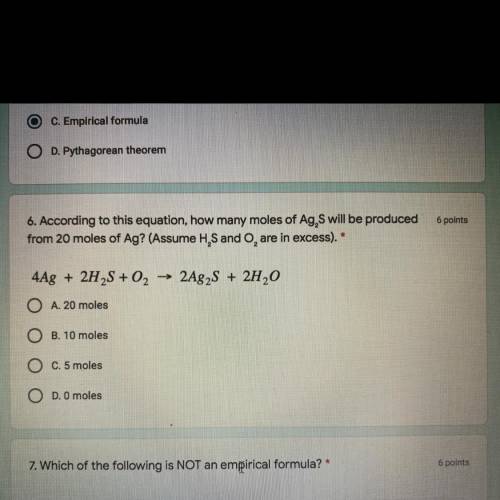
6 points
6. According to this equation, how many moles of Ag, S will be produced
from 20 mol...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Questions

Geography, 24.05.2020 00:05

Mathematics, 24.05.2020 00:05

Advanced Placement (AP), 24.05.2020 00:05

Geography, 24.05.2020 00:06


History, 24.05.2020 00:06


Mathematics, 24.05.2020 00:06

Mathematics, 24.05.2020 00:06

English, 24.05.2020 00:06

Mathematics, 24.05.2020 00:06


Mathematics, 24.05.2020 00:06


Mathematics, 24.05.2020 00:06


Social Studies, 24.05.2020 00:06

Mathematics, 24.05.2020 00:06

Mathematics, 24.05.2020 00:06