Cyclobutane decomposes to ethylene according to the equation
C4H8(g) → 2C2H4
Determine the o...

Chemistry, 24.01.2022 09:50 hurtadocrv
Cyclobutane decomposes to ethylene according to the equation
C4H8(g) → 2C2H4
Determine the order of the reaction and the rate constant based on the following pressures, which were recorded when the reaction was carried out at 430°C in a constant-volume vessel.
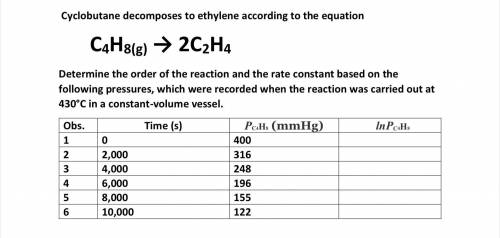

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
Questions

Biology, 10.11.2019 02:31

History, 10.11.2019 02:31


Health, 10.11.2019 02:31


Computers and Technology, 10.11.2019 02:31




Mathematics, 10.11.2019 02:31

Mathematics, 10.11.2019 02:31

History, 10.11.2019 02:31



Spanish, 10.11.2019 02:31



Computers and Technology, 10.11.2019 02:31





