
Chemistry, 31.01.2022 07:10 harleypage308
Please help!
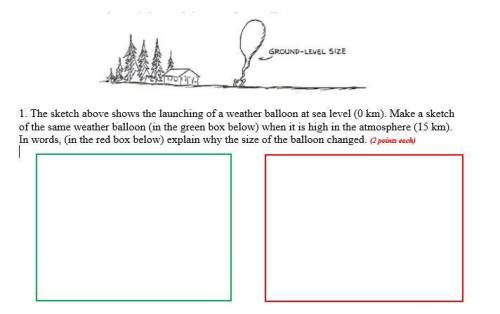
1. The sketch above shows the launching of a weather balloon at sea level (0 km). Make a sketch of the same weather balloon (in the green box below) when it is high in the atmosphere (15 km). In words, (in the red box below) explain why the size of the balloon changed
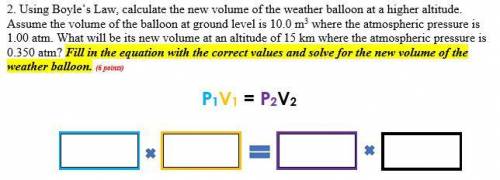
2. Using Boyle’s Law, calculate the new volume of the weather balloon at a higher altitude. Assume the volume of the balloon at ground level is 10.0 m3 where the atmospheric pressure is 1.00 atm. What will be its new volume at an altitude of 15 km where the atmospheric pressure is 0.350 atm? Fill in the equation with the correct values and solve for the new volume of the weather balloon.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Please help!
1. The sketch above shows the launching of a weather balloon at sea level (0 km). Mak...
Questions

Computers and Technology, 30.07.2019 17:30





Social Studies, 30.07.2019 17:30


Social Studies, 30.07.2019 17:30




Social Studies, 30.07.2019 17:30


Biology, 30.07.2019 17:30


Chemistry, 30.07.2019 17:30


Chemistry, 30.07.2019 17:30


Biology, 30.07.2019 17:30



