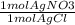Chemistry, 31.01.2022 18:50 valenzueladomipay09u
For the reaction represented by the equation AgNO3 + NaCl ® NaNO3 + AgCl, how many moles of silver chloride, AgCl, is produced from 7.0 mol of silver nitrate AgNO3?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
For the reaction represented by the equation AgNO3 + NaCl ® NaNO3 + AgCl,
how many moles of silver...
Questions


Health, 25.11.2021 17:50

Spanish, 25.11.2021 17:50


Mathematics, 25.11.2021 18:00





English, 25.11.2021 18:00




Health, 25.11.2021 18:00

Mathematics, 25.11.2021 18:00

History, 25.11.2021 18:00


Physics, 25.11.2021 18:00