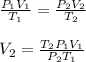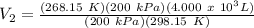Chemistry, 01.02.2022 14:00 Dsutton2021
4.000 x 10^3 L of propane gas is held in a tank at 25 °C. The tank has a moveable diaphragm to keep the pressure constant at 200 kPa. If the temperature falls to -5 °C on a cold winter day, what volume will the gas occupy?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3


Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 13:30
What would happen if no were added to n(g)+o2=2no(g) at equilibrium?
Answers: 1
You know the right answer?
4.000 x 10^3 L of propane gas is held in a tank at 25 °C. The tank has a moveable diaphragm to keep...
Questions





SAT, 24.12.2021 06:10

Mathematics, 24.12.2021 06:20





SAT, 24.12.2021 06:20



Mathematics, 24.12.2021 06:20

SAT, 24.12.2021 06:20

Mathematics, 24.12.2021 06:20

Computers and Technology, 24.12.2021 06:20

Mathematics, 24.12.2021 06:20


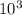 L
L