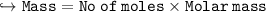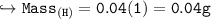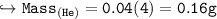Compare the masses of a one-liter sample of hydrogen and a one-liter sample of helium gas, each at 25°C and 5.0 atm pressure.
I will mark brainliest if right!!
A) the helium gas has twice the mass of the hydrogen gas
B) the helium gas has four times the mass of the hydrogen gas
C) the hydrogen gas has twice the mass of the helium gas
D) the hydrogen gas has four times the mass of the helium gas
E) the mass of the hydrogen gas equals the mass of the helium gas

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 23.06.2019 07:00
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3
You know the right answer?
Compare the masses of a one-liter sample of hydrogen and a one-liter sample of helium gas, each at 2...
Questions

Mathematics, 05.07.2019 22:30




Mathematics, 05.07.2019 22:30




Mathematics, 05.07.2019 22:30







Geography, 05.07.2019 22:30

Mathematics, 05.07.2019 22:30


Mathematics, 05.07.2019 22:30

Mathematics, 05.07.2019 22:30