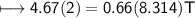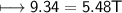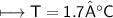Chemistry, 02.02.2022 04:00 nunnielangley0
What is the temperature (in K) of 0.660 mole of neon in a 2.00 L vessel at 4.68 atm?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
What is the temperature (in K) of 0.660 mole of neon in a 2.00 L vessel at 4.68 atm?...
Questions

Business, 21.11.2019 07:31

History, 21.11.2019 07:31

Mathematics, 21.11.2019 07:31


Computers and Technology, 21.11.2019 07:31

Mathematics, 21.11.2019 07:31



History, 21.11.2019 07:31


Biology, 21.11.2019 07:31

History, 21.11.2019 07:31

Mathematics, 21.11.2019 07:31

Social Studies, 21.11.2019 07:31


Biology, 21.11.2019 07:31




History, 21.11.2019 07:31