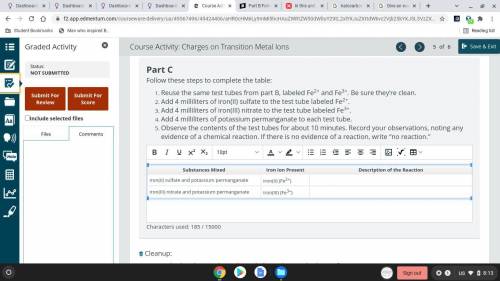
Part C
Follow these steps to complete the table:
Reuse the same test tubes from part B...

Chemistry, 03.02.2022 06:10 nforrestall
Part C
Follow these steps to complete the table:
Reuse the same test tubes from part B, labeled Fe2+ and Fe3+. Be sure they’re clean.
Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2+.
Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
Add 4 milliliters of potassium permanganate to each test tube.
Observe the contents of the test tubes for about 10 minutes. Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a reaction, write “no reaction.”

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
Questions

Spanish, 08.04.2021 23:10







World Languages, 08.04.2021 23:10


English, 08.04.2021 23:10






Mathematics, 08.04.2021 23:10

Mathematics, 08.04.2021 23:10

Mathematics, 08.04.2021 23:10




