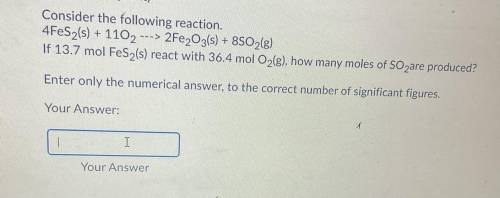
Consider the following reaction.
4FeS2(s) + 1102 ---> 2Fe2O3(s) + 8SO2(g)
If 13.7 mol FeS...

Chemistry, 07.02.2022 21:00 marley5818
Consider the following reaction.
4FeS2(s) + 1102 ---> 2Fe2O3(s) + 8SO2(g)
If 13.7 mol FeS2(s) react with 36.4 mol O2(g), how many moles of SO2 are produced?
Enter only the numerical answer to the correct number of significant figures.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

You know the right answer?
Questions


Mathematics, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01

Chemistry, 19.09.2020 01:01

Health, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01

English, 19.09.2020 01:01








Chemistry, 19.09.2020 01:01



Mathematics, 19.09.2020 01:01


Computers and Technology, 19.09.2020 01:01



