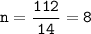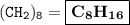Chemistry, 08.02.2022 14:00 tatilynnsoto17
A compound with the empirical formula CH2 was found to have a molar mass of approximately 112 g. Write the molecular formula of the compound.
2Points
Show all your work. Please use correct formatting for subscripts and exponents. The math formula editor makes it easier to show work.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
A compound with the empirical formula CH2 was found to have a molar mass of approximately 112 g. Wri...
Questions

Mathematics, 08.06.2021 05:10

Mathematics, 08.06.2021 05:10

Arts, 08.06.2021 05:10

Mathematics, 08.06.2021 05:10



Mathematics, 08.06.2021 05:10



Mathematics, 08.06.2021 05:10





Mathematics, 08.06.2021 05:10


Biology, 08.06.2021 05:10



Mathematics, 08.06.2021 05:10