
Chemistry, 08.02.2022 20:10 laqu33n021
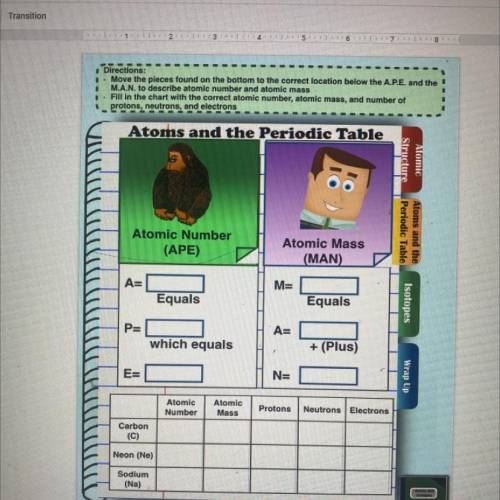
Directions
- Move the places found on the bottom to the correct location below the APE and the
• MAN. to describe tomic number and stoc mass
- Fin the chart the correct atomic number on maand umber of
protons, ons, and clectrons
Atoms and the Periodic Table
Atomic
Strasture Periodic Table
Atoms and the
Atomic Number
(APE)
Atomic Mass
(MAN)
A=
M=
Equals
Equals
Isotopes
P=
A=
which equals
+ (Plus)
-
Es
N=
Wrap Up
Atomie
Number
Atomie
Protons
Neutrons Electrons
Carbon
Neon
Sodium
Na
Neutron
Electron: Proton
menu
tommaso
Speaker notes

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 08:00
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Directions
- Move the places found on the bottom to the correct location below the APE and the
Questions

Health, 23.07.2019 00:30

History, 23.07.2019 00:30

Chemistry, 23.07.2019 00:30

Mathematics, 23.07.2019 00:30



Mathematics, 23.07.2019 00:30

History, 23.07.2019 00:30

Social Studies, 23.07.2019 00:30













