
Chemistry, 10.02.2022 20:10 mvasquez3122p4vahv
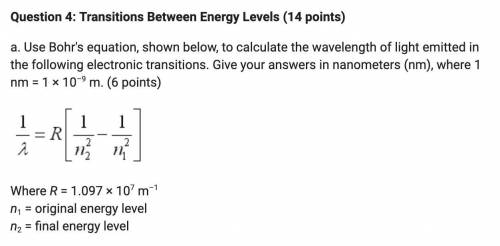
A. Use Bohr's equation, shown below, to calculate the wavelength of light emitted in the following electronic transitions. Give your answers in nanometers (nm), where 1 nm = 1 × 10–9 m.
i. n = 2 n = 1
ii. n = 4 n = 1
iii. n = 6 n = 1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
A. Use Bohr's equation, shown below, to calculate the wavelength of light emitted in the following e...
Questions



History, 12.06.2020 04:57

Mathematics, 12.06.2020 04:57


Health, 12.06.2020 04:57







Mathematics, 12.06.2020 04:57

Social Studies, 12.06.2020 04:57




Mathematics, 12.06.2020 04:57

Mathematics, 12.06.2020 04:57



