Magnesium + oxygen
2Mg(s) + O2 (9)
magnesium oxide
2MgO (s)
1. Using the equatio...

Chemistry, 11.02.2022 14:00 kayranicole1
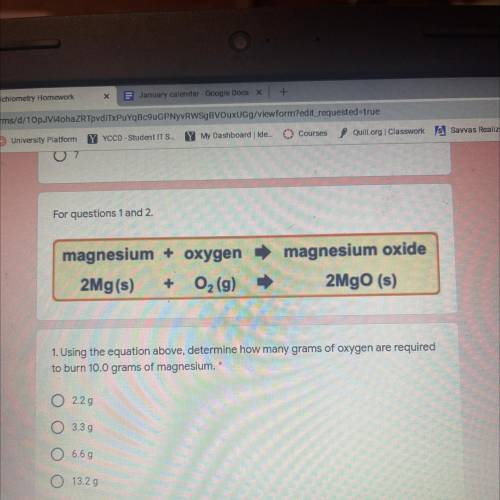
Magnesium + oxygen
2Mg(s) + O2 (9)
magnesium oxide
2MgO (s)
1. Using the equation above, determine how many grams of oxygen are required
to burn 10.0 grams of magnesium.
2.29
O 3.39
6.69
Ο Ο
13.29

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1


Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
Questions


Mathematics, 22.01.2021 20:50


Chemistry, 22.01.2021 20:50


English, 22.01.2021 20:50


Mathematics, 22.01.2021 20:50



Mathematics, 22.01.2021 20:50


Arts, 22.01.2021 20:50

Mathematics, 22.01.2021 20:50

Mathematics, 22.01.2021 20:50



Mathematics, 22.01.2021 20:50


Advanced Placement (AP), 22.01.2021 20:50



