Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
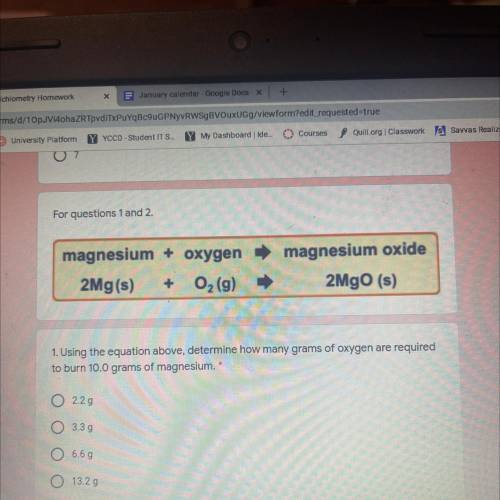
Magnesium + oxygen
2Mg(s) + O2 (9)
magnesium oxide
2MgO (s)
1. Using the equatio...
magnesium oxide
2MgO (s)
1. Using the equatio...
Questions

Computers and Technology, 17.08.2020 22:01

Chemistry, 17.08.2020 22:01


Chemistry, 17.08.2020 22:01




English, 17.08.2020 22:01

Mathematics, 17.08.2020 22:01

English, 17.08.2020 22:01


Chemistry, 17.08.2020 22:01

Mathematics, 17.08.2020 22:01

Mathematics, 17.08.2020 22:01


World Languages, 17.08.2020 22:01