TARGET MASS OF Pbl2, 8 (given by lab instructor)
colo
g
You will be given both reactan...

Chemistry, 11.02.2022 17:00 EliHarris517
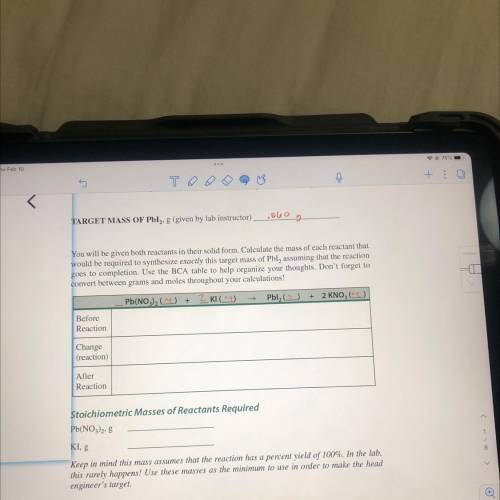
TARGET MASS OF Pbl2, 8 (given by lab instructor)
colo
g
You will be given both reactants in their solid form. Calculate the mass of each reactant that
would be required to synthesize exactly this target mass of Pbl, assuming that the reaction
goes to completion. Use the BCA table to help organize your thoughts. Don't forget to
convert between grams and moles throughout your calculations!
Pb(NO3)2 (
+
2 Klag)
Pbl (5)
+ 2 KNO (2)
Before
Reaction
Change
(reaction)
After
Reaction

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1


Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Questions


Mathematics, 27.10.2020 22:10

Mathematics, 27.10.2020 22:10

Advanced Placement (AP), 27.10.2020 22:10

English, 27.10.2020 22:10




Mathematics, 27.10.2020 22:10

Mathematics, 27.10.2020 22:10


Biology, 27.10.2020 22:10




Mathematics, 27.10.2020 22:10


Mathematics, 27.10.2020 22:10




