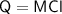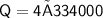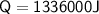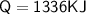Chemistry, 11.02.2022 20:40 micahwilkerson9495
Calculate the heat energy required to melt 4kg of ice when the specific latent heat of fusion of water is 334,000 J/kg.
1336 kJ
2336 kJ
3336 kJ
HELP ASAP

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1


Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
Calculate the heat energy required to melt 4kg of ice when the specific latent heat of fusion of wat...
Questions





Mathematics, 24.04.2020 22:47

History, 24.04.2020 22:47


Mathematics, 24.04.2020 22:47



History, 24.04.2020 22:47

Chemistry, 24.04.2020 22:47

Mathematics, 24.04.2020 22:47

Mathematics, 24.04.2020 22:47


History, 24.04.2020 22:47

Mathematics, 24.04.2020 22:47


English, 24.04.2020 22:47

Mathematics, 24.04.2020 22:47