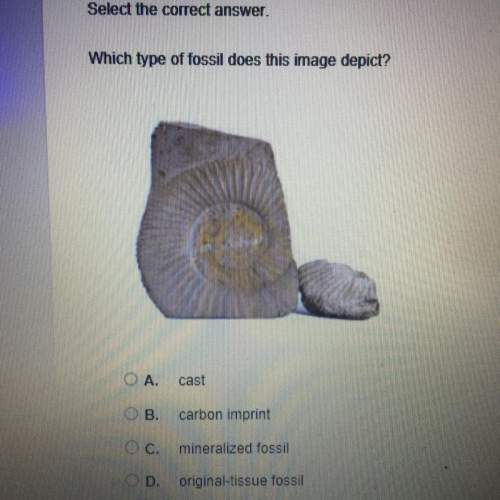
TARGET MASS OF Pbl2, 8 (given by lab instructor)
colo
g
You will be given both reactan...

Chemistry, 11.02.2022 22:00 destineenikole17
TARGET MASS OF Pbl2, 8 (given by lab instructor)
colo
g
You will be given both reactants in their solid form. Calculate the mass of each reactant that
would be required to synthesize exactly this target mass of Pbl, assuming that the reaction
goes to completion. Use the BCA table to help organize your thoughts. Don't forget to
convert between grams and moles throughout your calculations!
Pb(NO3)2 (
+
2 Klag)
Pbl (5)
+ 2 KNO (2)
Before
Reaction
Change
(reaction)
After
Reaction
I’m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
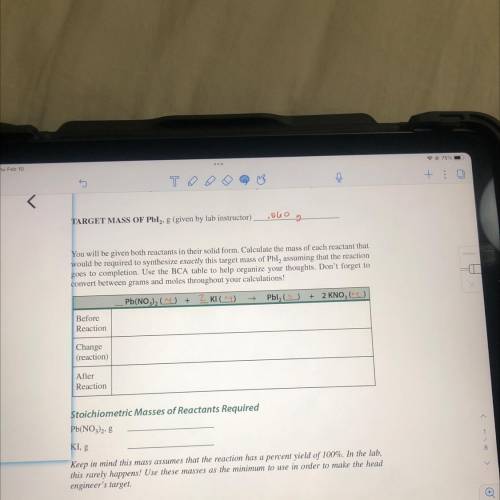
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
Questions

Mathematics, 23.09.2019 05:00

Mathematics, 23.09.2019 05:00


Mathematics, 23.09.2019 05:00




History, 23.09.2019 05:00

Arts, 23.09.2019 05:00



Physics, 23.09.2019 05:00

Social Studies, 23.09.2019 05:00


Chemistry, 23.09.2019 05:00



Social Studies, 23.09.2019 05:00

Social Studies, 23.09.2019 05:00