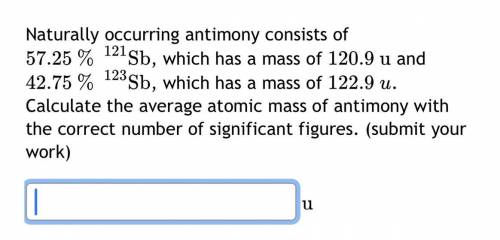
Naturally occurring antimony consists of
57.25
%
121
Sb
57.25
...

Naturally occurring antimony consists of
57.25
%
121
Sb
57.25
%
121
Sb
, which has a mass of
120.9
u
120.9
u
and
42.75
%
123
Sb
42.75
%
123
Sb
, which has a mass of
122.9
u
122.9
u
. Calculate the average atomic mass of antimony with the correct number of significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which statement is a reason to support population regulation? a) it is unethical for us to control birth control rates b) humans have the freedom to produce as many children as desired c) the gap between the rich and poor has been narrowing since 1960 d) billions more people on the earth will intensify many environmental and social problems
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
Questions



Mathematics, 23.06.2020 10:57


Mathematics, 23.06.2020 10:57

History, 23.06.2020 10:57



Mathematics, 23.06.2020 10:57





Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57


Mathematics, 23.06.2020 10:57

English, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57



