
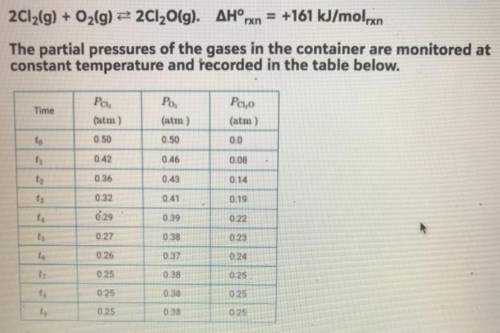
Equimolar amounts of Cl2 (g) and O2 (g) are injected into an evacuated, rigid container, where they react according to the equation below (see picture)
a. if 6.4 g of O2 (g) is consumed in the reaction with excess Cl2 (g), how many moles of Cl2O (g) are produced?
b. Which element is oxidized in this reaction? Justify your answer in terms of oxidation number.
c. At time t4, is the rate of the reverse reaction greater than, less than, or equal to the rate of the forward reaction? Justify your choice.
d. At equilibrium, the container holds fewer molecules of which gas, Cl2 (g) or O2 (g)? Explain your answer.
e. A student hypothesizes that if the temperature of the container is decreased after time t9, the mole fraction of Cl2O (g) in the container will increase. Do you agree or disagree with the student's hypothesis? Justify your answer.
f. Using the data in the table, determine the value of the equilibrium constant, Kp, for the reaction represented by the equation below.
2 Cl2 (g) + O2 (g) <-> 2 Cl2O (g)
g. Using the value determined in part (f), determine the value of Kp for the equation shown below.
4 Cl2 (g) + 2 O2 <-> 4 Cl2O (g)
h. Which gas, Cl2 (g) or O2 (g), will deviate most from the ideal gas law at low temperature? Justify your choice.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
How many miles of calcium oxide will be produced when 1.6 miles of iron (iii) oxide react with calcium phosphate
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Equimolar amounts of Cl2 (g) and O2 (g) are injected into an evacuated, rigid container, where they...
Questions

Social Studies, 02.02.2020 06:42



History, 02.02.2020 06:42

Biology, 02.02.2020 06:42

Social Studies, 02.02.2020 06:42






Mathematics, 02.02.2020 06:42



History, 02.02.2020 06:42


Mathematics, 02.02.2020 06:42





