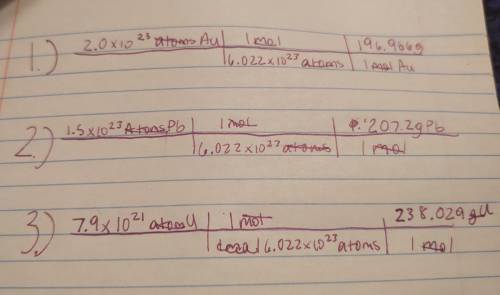Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
PLEASE HELP FAST !
Calculate the mass in grams of each of the following . 2.0•10^23 gold atoms 1.5...
Questions

English, 30.06.2021 17:40

Mathematics, 30.06.2021 17:40


Mathematics, 30.06.2021 17:40


Mathematics, 30.06.2021 17:40


Mathematics, 30.06.2021 17:40


Mathematics, 30.06.2021 17:40

Chemistry, 30.06.2021 17:40


Mathematics, 30.06.2021 17:40




Mathematics, 30.06.2021 17:40

Advanced Placement (AP), 30.06.2021 17:40

Computers and Technology, 30.06.2021 17:40

Mathematics, 30.06.2021 17:40